Water
Article curated by Ginny Smith
Without water, life as we know it couldn't exist. But despite its abundance, it remains a mysterious substance.

Properties of water
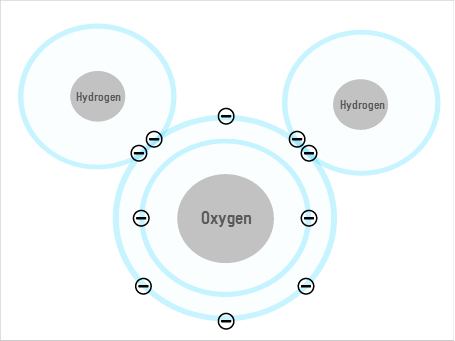
Scientists discovered the componants of water in the late 18th Century, and it seems like a simple molecule – just two hydrogens and an oxygen. But it has some unusual properties – like that ice floats on water and that the surface tension lets insects to walk on it. These properties don't seem strange to us: we're so used to seeing water(!), but when compared to other liquids it becomes clear how unusual water is. If, for example, you have ever left a bottle of olive oil somewhere cold, you will know that when oil freezes, the solid sinks in the liquid. Water is the odd one out.
Some of water's unusual properties come from the fact that its shape allows it to form hydrogen bonds. The oxygen atom has a slightly negative charge (or "dipole"), and the two hydrogens are slightly positive, so the oxygen of one molecule can attract the hydrogen of the next, forming a lattice of multiple molecules. But while this may explain some of water's weird behaviours, it doesn't explain everything.

Water freezing to ice is something we're all familiar with, but there are still elements of the process that are mysterious. One example is the Mpemba Effect – the fact that, perhaps counter-intuitively, hot liquids freeze faster than cold ones!
Explanations have been suggested – evaporation releases heat, and the warmer liquid’s higher rate of evaporation would reduce the mass that's freezing. Warmer liquids will also have more active convection currents, which could release more heat and cool the liquid faster. And the act of heating the water might have changed the impurities and solutes in it, changing the rate of cooling. However, none of these things have a big enough effect on the rate of cooling to explain the Mpemba effect.
More complex explanations have also been hypothesised – many involving supercooling. Supercooling happens when something is cooled to past its freezing point but doesn’t freeze because there is nothing for the ice crystals to form around. In 1994, experiments showed that water that started out hot supercooled less than initially colder water.
Learn more about The Mpemba Effect.

 2
2

Learn more about The phases of water as it freezes.

 2
2Another unusual property of water is that it is at its most dense at 4 degrees Celsius. Unlike most other liquids, water becomes less dense as it freezes, thanks to hydrogen bonding holding apart the molecules in solid ice. However, why max density is 4 degrees Celsius rather than any other temperature is not clear.
Learn more about Why is water most dense at 4 degrees Celsius?.

 2
2The critical point of a substance is the point where it can exist in two different forms, for example, as a liquid and a gas (otherwise known as being in equilibrium). The critical point of water is incredibly high compared with other molecules of the same structure, for example H2S or H2Se. This is likely to be partly due to hydrogen bonding, and formation of interconnected networks of water molecules, but is not understood.

Water in space
For life as we know it to be viable, water is a necessity. We humans are composed of 60% water and can't survive more than a few days without it. The level of dependence does vary from species to species, however every organism we've discovered has some dependence on it.
When searching for bodies that may be able to support life, scientists first look for Earth-like planets with liquid surface water. However, just because it is the case on Earth does it mean this is true universally? We don't know. It is possible that life forms have developed on other planets that have different needs because of alternative biochemistries and need other kinds of liquids.
Learn more about dependency of life on water.

 2
2
The answer lies in detecting present rates of water loss and trying to establish past activity. While hydrogen and oxygen have been measured escaping from the side of the planet facing away from the sun in a two to one ratio (the same as that found in water), so far only hydrogen has been detected being stripped from the day side of the atmosphere (the side facing the sun). So far, there is no proof that water is the source of these gases, and we can't be sure that water existed on Venus.

How much water is there on Mars? Mars’ atmosphere contains very little oxygen, and its surface has little to no liquid water. However, we can clearly identify polar ice caps on Mars using ground based telescopes, and measurements made by the Mars Express satellite and the Mars Reconnaissance Orbiter have been able to largely quantify what portion of these is water ice (the majority of the rest being frozen carbon dioxide). Any other water must be trapped in or beneath the rock, and is difficult to find and measure.

 3
3![Water on the moon By Credits: ISRO/NASA/JPL-Caltech/USGS/Brown Univ. [Public domain], via Wikimedia Commons](/img/sci/Chandrayaan1_Spacecraft_Discovery_Moon_Water.jpg)
Unfortunately, these tentative results will be extremely difficult to confirm, and samples are needed. These are challenging to obtain, especially on such rugged terrain.

Europa, one of Jupiter's moons, may also have water. Scientists have reported water flowing up through the cracks in the surface caused by asteroid impacts. However, temperatures on Europa’s surface range between 53K and 111K, much to cold for liquid water to exist, and scientists suggest it is covered in a sheet of ice with liquid water underneath[1][2]. The water is thought to be kept liquid either because of a volcanic inner layer or tidal heating due to Jupiter's gravitational effects.

 2
2
But is it clean, or salty? A yellow-brown colour suggests the presence of magnesium sulfate, but it could instead by irradiated sodium chloride, which we have not been able to detect yet as it is “spectrally inactive” over much of the range used in remote sensing. If the salts are of internal origin, this suggests interactions between Europa’s ocean and a “silicate seafloor”, which is considered critical in assessments of habitability.


Ganymede, Jupiters largest moon, has been known to have an ocean hundreds of miles deep since NASA's Galileo mission flew by. Theoretical evidence suggests it may be composed of several layers of salty waters and ices. Using computerised simulations and models, scientists have concluded that a likely composition of is water sandwiched between up to three ice layers, with a salty liquid layer next to the rocky sea floor. Researchers are searching for evidence that this computer simulation is true.
Learn more about The composition of Ganymede.

 3
3Saturn's moon Enceladus had been identified as having water ice on it's surface even before the voyager flybys of the 1980s. More recently, The Cassini mission observed enormous plumes of water ice, containing some organic molecules being ejected from the moon near it's southern pole. The question here is where this water ice comes from, it is possible that there are liquid pockets or even a world spanning liquid water ocean under the ice, but without sending instruments to land on Enceladus itself and get through the surface ice, we will not be able to tell if this is the case.

 2
2Water on Earth

Learn more about Origin of Earth's water.


 2
2
How the chemistry of seawater and the atmosphere have changed during and since the early evolution of life on our planet, its driving forces and biological feedbacks remain controversial. Understanding the mechanisms controlling the availability of bioessential elements (e.g., Fe and Si) in predominantly anoxic, ferruginous Precambrian seawater is critical to our understanding of the evolution of prokaryotic and eventually eukaryotic life. Researchers at the University of Oxford are exploring this using experimental geochemistry, crystallography, and micro-petrology to establish the mineralogical, trace element and isotopic fingerprints that reveal the links between mineral stability and environments.


Various phenomena are seen on earth due to water. One of them is superterminal traindrops – raindrops that fall much faster than they really should (30% faster than their terminal velocity). It’s not yet clear why these drops are falling faster than expected, but it's possible that the speedy drops are fragments of larger drops that have broken apart in midair but have yet to slow down. This would illustrate that raindrop disintegration happens far more often than previously thought. It may be due to raindrops becoming unstable after collisions, however further studies are necessary.

 2
2Another phenomenon is the glory: a concentric ring of colours, looking like a circular rainbow. It is most often seen on a Brocken spectre – the projection of a magnified shadow that falls on clouds (water droplets) when they are below the observer with the sun behind them. Although it is similar to a rainbow, scientists think that the origin of the glory is different. Two current theories include (1) the Hendrik van de Hulst model involving surface wave interference between those taking long and short paths after entering droplets at different points and being internally reflected; and (2) the Herch Moyses Nussenzveig theory of classical wave tunnelling, which occurs when evanescent light waves travel both on the surface of a drop and inside it.


 2
2The Earth's water cycle is a complex system, with a change in one variable often affecting many others. Predicting how rising global temperatures will affect the quantity and pattern of rainfall, for example, is extremely challenging, particularly on a local scale. Many models suggest that even if global rainfall increases, dry areas may become drier, which is bad news for animals, plants and people in these areas. Other areas, however, are likely to get wetter, with floods becoming more common. One thing we do know for sure is that rising temperatures will cause changes that will affect us all. It's vital we try and improve our predictions to help avoid what could be devastating local effects of climate change.

 4
4
Rising sea levels could cause many problems – the most obvious being that areas which are currently occupied by humans, animals and plants will end up underwater and uninhabitable. There may also be stronger storms that could devastate coastal regions. Unfortunately, predicting these effects is challenging – there are many factors that influence sea level.
Learn more about Rising Sea Levels.

 3
3
Life in the oceans


 2
2One by-product of global warming is the melting of glaciers, which provides more fresh water, boosting the number of living things in the area around the glacier. While this may seem like a good thing, research has shown that it also reduces the diversity of the plants and animals nearby, putting cold-loving extremophiles at risk of extinction.


 3
3Thirst
We don't actually know what factors – salt, temperature – regulate thirst in humans, nor how.

Does salt make you thirsty? Eating table salt by itself is almost always be followed by a drink of water, but the assumption that consuming these foods increases thirst has been challenged. It found that there was no correlation in their test group for salt consumption and drinking water. Though it was a small test group (58) and a specific age range (students).

We know that the mineral content of water varies with rocks in different regions and that mineral content is vital for our health, from our bones to our hair to our energy levels and sun tolerance. It also changes the taste of the water. As such, some people don’t like the water in certain regions, and “good” water is treasured and sold in bottles. It’s also used in manufacturing to give that distinctive flavour. Scottish distilleries, for example, treasure their local water sources and attribute them responsible for their brand’s distinctive flavours. However, we don’t even know if you can still taste those flavours after chemical manufacturing processes such as distillation. Minerals could also affect process such as the productivity of yeast.


 2
2Clean water





These substances haven't been around for long enough for us to know if low level constant exposure is harmful. Recent studies in fish have shown that trace amounts of certain psychoactive drugs (drugs designed to affect the brain) can produce real side effects. The amounts in drinking water are most likely too small to affect adults, but there could potentially be some risk to unborn children when pregnant women are exposed. However, it remains unclear whether the quantities of these drugs which make it into the water supply are significant enough to have any effect at all. Studies are yet to be published involving the reaction of mammals, let alone humans, to doses at this level.


Pollution can have a negative impact on wildlife. In some cases, animals may realise the water is unusable so they have to travel further or compete with others for remaining usable sources. In other cases, the pollution may be more subtle, so they animals don't notice it and continue using the water. This can have long term health effects – mercury poisoning, for example, is thought to cause problems in young animals. Studying how different pollutants may affect different species is difficult as natural variables are impossible to control, and are sometimes unknown, but it is vital if we are to prevent further harm being done to our wildlife.


Learn more about The impact of microplastics on sea animals.


 3
3We really don't know what microorganic pollutantsare in our water, especially in low to middle-income countries and those outside of North America and Western Europe. However, overviews of Global Detection rates appear to show that contaminants including illicit drugs occur more in affluent First World locations.


Trace contaminants can be really, really hard to detect because they occur in such small amounts. They tend to form amorphous or mixed phases, colloids, polymers, or dissolve in water – all phases which are inscrutable using analytical techniques such as x-ray diffraction, which identifies crystalline phases. Even when their total content may be quantified, their chemical environments are only scrutable if they persist in large fractions. Most research focuses on major contaminants, and lots of stuff gets ignored. Scarily, side effects that might include the mobilisation of other, more toxic, trace materials become important, and may dominate over the results of remediation.


New ways to provide drinkable water sources are under development. In many places, water is abundant, but it is unusable because it is salty. Desalination plants, which convert sea water into fresh water, are increasingly being built, but require a lot of energy. Small devices such as watercones use solar energy to purify salty or brackish water; these are very useful to countries with a very small amount of available water such as Africa. However many people are still looking for one truly satisfactory way of providing water; it must be cheap, not require a lot of energy and must provide enough clean water for our daily needs.




 2
2Contaminants vary widely, including antibiotics, azo dyes, chlorinated solvents and pesticides, nitro compounds, organophosphates, phenyls and heavy metal ions. Removal mechanisms include sorption, degradation, complexation, precipitation and reduction, but remain challenging and varied. That iron nanoparticles provide a low cost, easily manufactured way of removing rogue contaminants from the environment is very exciting; however, researchers have attempted to develop an understanding of the patterns which govern choice of reaction with iron nanoparticles, but the chemistry remains complex and highly dependent upon conditions. Other problems include degradation products, if the contaminants or nanoparticles break down, nanotoxicity, and problems with tracking and tracing contaminants.


Other filters include zeolites, graphene oxide (the morphological equivalent of a crumpled piece of paper), titanium dioxide nanoparticles, and phytoremediation, using plants! These avoid harsh chemical cleaning conditions.


Scientists are still working out how to police environmental remediation and safe cleanup, without simply moving contaminants on or starting a blame game (because cotaminants drift). Large scientific remediation projects like geoengineering to combat the effects of anthropogenic climate change will also effect different places in different ways, causing droughts and floods and uneven heating and cooling to average a lower global temperature – there are winners and losers… so who decides? These effects are hard to understand, difficult to predict and politically awkward to legislate.


 3
3This article was written by the Things We Don’t Know editorial team, with contributions from Ed Trollope, Jon Cheyne, Freya Leask, Ginny Smith, Cait Percy, Johanna Blee, Grace Mason-Jarrett, Kat Day, Rowena Fletcher-Wood, Joshua Fleming, Holly Godwin, and Alice Wayne.
This article was first published on 2016-01-21 and was last updated on 2020-08-10.
References
why don’t all references have links?
[1] Kivelson, M.G., et al., (2000) Galileo Magnetometer Measurements: A Stronger Case for a Subsurface Ocean at Europa. Science 289(5483):1340-1343 DOI: 10.1126/science.289.5483.1340
[2] Carr, M.H., et al., (1998) Evidence for a subsurface ocean on Europa. Nature 391:363-365 DOI: 10.1038/34857
[3] Hallis, L., et al., (2015) Evidence for primordial water in Earth’s deep mantle Science 6262/ 350:795-797 DOI: 10.1126/science.aac4834
[4] Sarafian, A. R., et al, (2014) Early accretion of water in the inner solar system from a carbonaceous chondrite–like source Science 6209/ 346:623-626 DOI: 10.1126/science.1256717
[5] Shebek, K., et al., (2015) The Flocculating Cationic Polypetide from Moringa oleifera Seeds Damages Bacterial Cell Membranes by Causing Membrane Fusion Langmuir 31.15:4496–4502 DOI: 10.1021/acs.langmuir.5b00015
Blog posts about Water






Recent Water News
Get customised news updates on your homepage by subscribing to articles