Ambient chemistry
Article curated by Rowena Fletcher-Wood
Chemistry is forever learning its lessons from nature. Nature has been performing chemical reactions since well before the first living things. Now it performs most of its biochemical reactions under similar temperatures and conditions - body temperature. It’s amazing anything happens at all: even geological processes, performed under extreme temperatures and pressures, happen slowly, over hundreds, thousands, and millions of years. But the molecules found in our bodies are more delicate and reactive than rock crystals. Yet somehow in the mishmash of many molecules in the body, it manages to build complex systems selectively on a pretty small energy input. It uses various tricks to do this, including using metal-organic compounds and handed molecules (molecules with a distinct mirror image).

Metal-organic compounds
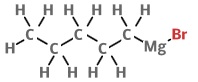


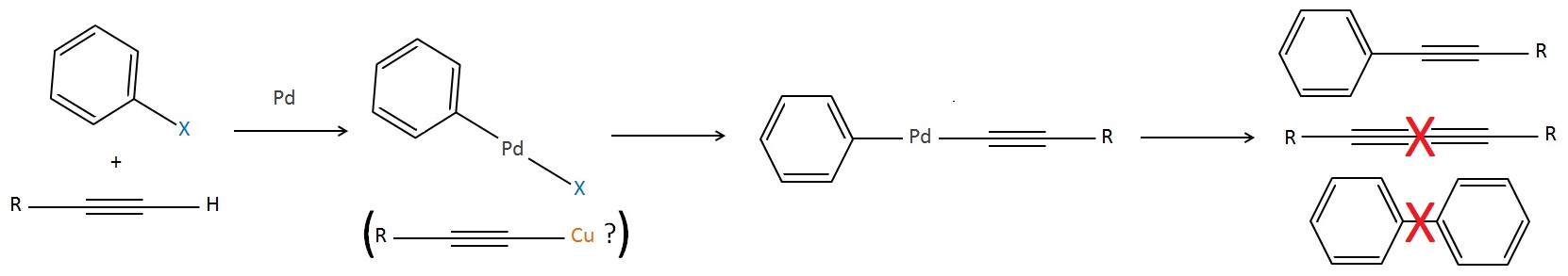
Sonogashira reaction Palladium is a common metal organic catalyst. Chemists think palladium inserts into weak bonds like carbon-halide bonds, then later swaps or deinserts, creating new pairs. Some of these intermediates have been isolated by cooling the liquid and crystallising them out as solids, but the full mechanisms aren’t known for many reactions, in particular, the Sonogashira reaction, which describes a reaction that links an alkyne to an organic halide using palladium. Some chemists think small amounts of copper contaminating the reaction are essential for making it work, but it’s difficult to prove them right or wrong, as copper impurities in palladium tend to be extremely hard to get rid of. The selectivity of the reaction is also hard to justify: the alkyne always pairs with a non-alkyne; you never get two non-alkynes joined together, or two alkynes - why? Some researchers think the Sonogashira reaction proceeds by one of two competing mechanisms [1], depending on how electron-withdrawing or electron-donating the organic parts are. Research is ongoing. Understanding more about these reactions could help chemists find even better alternatives. For example, palladium is a heavy metal, toxic to living things; and, no matter how careful you are, some will always contaminate products and equipment. Chemists want to find ways to replace it with other elements like silicon.


Handed molecules
Handedness molecules are (usually small) molecules with a mirror image that isn’t the same as itself. Like a pair of hands, you can’t turn one to look like the other: you can always distinguish left from right. Organic materials are mostly made of carbon, which forms 4 bonds. If each of the four bonds is to something different, the molecule will be handed. Although the groups spread out equally, forming the points of a triangular based pyramid (or tetrahedron), there is no way to turn one mirror image round until it looks like the other: they are non-superimposable. Some molecules have more than one carbon in them that is bonded to four different things. These molecules are not always mirror images of each other because the first centre could be right- or left-handed and so could the second, allowing for right-right, right-left, left-right and left-left possible molecules. The handed carbons are known as chiral centres. Chirality provides subtle control over biological processes because nature is inherently asymmetric down to a molecular level (although we don’t know why). All natural sugars and amino acids are chiral. As such, only left-handed versions of important molecules like enzymes can bind to receptors to send signals and instructions around the human body: the right-handed versions just don't “fit” - and are never synthesised by the body. If they were, they’d merely dilute the effect of the left-handed pair and slow down reactions. Alternatively, right-handed enzymes or drugs might fit completely different receptors elsewhere in the body, causing different, unintentional outcomes. The most famous example of the dangers of introducing wrong-handed molecules in a drug is thalidomide. Thalidomide was used to treat morning sickness in pregnant women, but initially it wasn't recognised that only one handed pair had this effect... the other caused serious birth defects. Some chiral molecules can send signals in the body that are misleading, like left-handed L-glucose. Both right- and left-handed glucose taste sweet, but our handed bodies can only metabolise the right-handed, naturally occurring sugar D-glucose. This means that L-glucose acts as a zero-calorie artificial sweetener, and gets washed out of the body unprocessed. Making chiral molecules is hard. Chemical reactions tend to be random, governed by the diffusion of molecules through solution, how often they collide, how hard, and at what angle. When reactions happen at random you get an even mixture of right- and left-handed molecules. We still don’t know quite how the body creates handedness, but since they do, chemists really want to make handed molecules for medicinal applications. Yet there exists no definitive known method for preparing singly-handed compounds from scratch. This challenge is so great it forms its own chemical field: the field of asymmetric synthesis. There are two ways to make chiral molecules: make a mixture and separate it out, or make only one of the chiral pair.

 2
2


Asymmetric synthesis In order to make more of one handed molecule than its pair, there needs to be a difference in the energy cost of making them. This only happens when starting materials or catalysts are chiral. Once you have a chiral centre, there are various reactions that can be used to manipulate it without creating a mixture. The best simple way to control the chemistry of a chiral centre is to attach a big bulky group to the "wrong" side of the molecule, restricting the access of incoming reactants. This doesn't always give 100% singly-handed products, but it does skew the balance a lot. Many different methods exist to synthesise specific asymmetric molecules under specific conditions. Internal mechanisms, such as the formation of circles when a molecules bites itself on the bum, may also be selective: big groups stick out where there is most space, and small groups get crowded together in the middle. Sometimes reactions take place inside other materials, such as porous solids that can act like catalysts. Pores of certain sizes and shapes may restrict the formation of some intermediates and allow others. For big, branched molecules, this method can be used to introduce selective chirality. Organometallics are sometimes used as catalysts. Unfortunately, most asymmetric syntheses still involve mind bogglingly large numbers of steps and very small final product yields.



Soft chemistry
Organometallics and solid catalysts used for asymmetric synthesis are themselves increasingly formed under ambient conditions, temperatures no more than 500oC (and usually lower than 200 oC) using a method called "chimie douche" or “soft chemistry” which mimics biological conditions rather than geological ones (“hard chemistry”) to make solid materials. This branch of chemistry only evolved in the 1980s, draws on the capacity of nature to build from simple starting materials using highly selective sequences. The results are products that would decompose under harsher conditions, but show enhanced flexibility, sensitivity and functionality. Soft chemistry is topotactic: maintaining some structural components, rather than bashing the whole thing up and starting from scratch (hard chemistry). Instead, it involves getting inside solid structures and tampering with them. In a way, it’s a bit like making a trifle: layer up the cake, fruit, jelly, custard and cream to make distinct layers with different flavours. But all the ingredients together before you bake, and you would simply make a rather unusual fruitcake. Existing “soft” materials include molecular sieves used to purify air and make oxygen in hospitals, biocements for fixing broken bones, luminescent materials used as biological tracers, and minerals with funky electrical and magnetic properties, such as superconductors. Soft chemistry usually makes inorganic materials, but combined organic-inorganic structures have also been made, such as metal organic frameworks (MOFs), which can be used for sensing and drug delivery. Soft chemistry isn’t foolproof, however. Bad soft chemistry often produces mixtures of products and methods are still being explored and uncovered. Methods The sol-gel method uses small molecules to build up large, repeating solid structures in the same way that organic polymerisation produces plastics from hydrocarbons - by giving the components exactly the right set of instructions. Hydrothermal synthesis involves using ordinary oven temperatures and a pressure autoclave (or bomb calorimeter)containing liquid-solid slurry of starting materials. Despite the exciting name, nothing is exploded. The bomb consists of a Teflon liner and metal case that is screwed tightly shut. As the temperature gets above the boiling point of the solvent (100 oC for water), it vaporises. As gas takes up more space than a liquid, pressure builds up and drives the crystallisation of novel solids. Other scientists are using microwave heating to create similar conditions. Intercalation is the process of inserting things between layers in a material such as graphite. A good way to intercalate something into a solid is to flow a gas over the solid for a long time under gentle heating. Often, the gas will percolate through the cavities in the solid and end up resting in the interlayer spaces. This could be a way to store hydrogen for hydrogen fuel cells. The bonds between intercalated materials and the host material tend to be weak and would not survive under harsher conditions. You can also deintercalate, removing small molecules from layers, such as taking out water (dehydration) to make new, open “metastable” structures. Pillaring involves slowly intercalating more and more things between layers to see how far you can go. Intercalating or deintercalating too many things between layers can weaken the bonds between the layers themselves, allowing them to slip apart and form thin nanomaterial sheets that may exhibit unusual properties because of their smallness. Doping processes intercalate or deintercalate charge carriers, creating a patchy solid full of defects and a unique electronic makeup. Because solid materials are so big and contain so many atoms, the properties we measure are a reflection of the average.


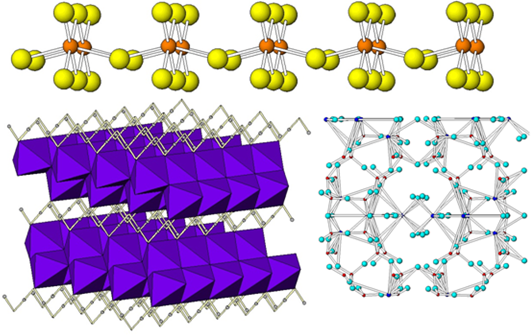
Despite these and many other new chemical methods, chemistry is forever learning from nature. The complexity and energy efficiency of natural chemical reactions, and the ability of nature to introduce successive ingredients as they are needed, means that they represent a pinnacle of chemical achievement. Although we can make organometallics, chemists still don’t know how to make ones as structurally complex as haemoglobin. They’d love to find a new method of making single-handed chiral molecules that works every time, but just haven't got there yet. And they can use soft chemistry to mimic biological conditions, but low temperatures impose limitations on the rate and extent of reactions, making reactions hard to scale up and industrialise. Overcoming these challenges, could enable chemists to tailor design future materials to fit the needs of the developing world. But don’t worry, they’re still evolving.
This article was written by the Things We Don’t Know editorial team, with contributions from Freya Leask, and Rowena Fletcher-Wood.
This article was first published on 2020-11-25 and was last updated on 2020-11-25.
References
why don’t all references have links?
Recent ambient chemistry News
Get customised news updates on your homepage by subscribing to articles